Why It Matters
Carbon dioxide gas dissolves so readily in seawater that approximately one quarter of human caused CO₂ emissions become sequestered in the ocean. Once in the ocean, CO₂ combines with water to form a weak acid, resulting in a change in the chemistry of the sea.
The Chemistry of Ocean Acidification
The other major change in seawater chemistry involves CO₂-driven changes in the solubility of calcium carbonate minerals (CaCO₃) used by many marine plants and animals to build their shells and skeletons.rnrnThe solubility of CaCO₃ minerals depend on the amount of dissolved carbonate ions in seawater.rnrnMore CO₂ and lower pH reduces the concentration of carbonate ions, making it more difficult for many organisms to make shell material.
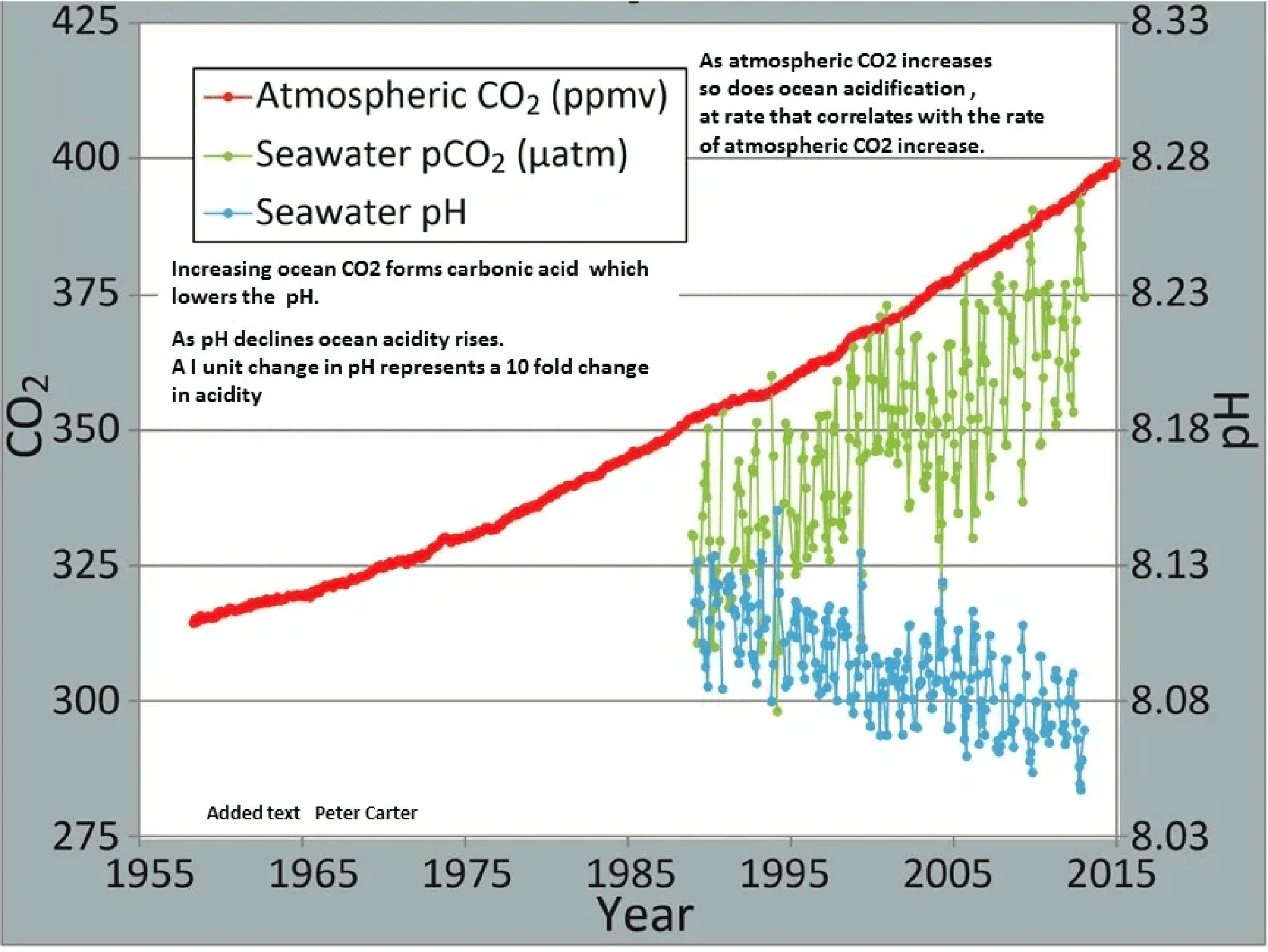
Ocean CO₂ and pH from NOAA: Correlation between rising levels of CO2 in the atmosphere at Mauna Loa with rising CO₂ levels in ocean at Station Aloha. As the CO₂ increases, the pH decreases; indicating increasing acidificication.

Ocean Acidification Is One of Many Human Impacts Changing Marine Ecosystems
Other human-influenced stressors, such as nutrient-induced oxygen deficiencies (hypoxia) and rising water temperatures, co-occur with normal daily and seasonal marine cycles like salinity , primary productivity, and tides. These interactions, influenced by both natural processes and changes in climate, resulting in complex and often unpredictable ecological responses.
The Interaction of Multiple Stressors
Multiple stressors interact and affect individual organisms and entire ecosystems in a complex way. The impact of co-occurring stressors can be additive or synergistic (meaning that the combined effect is greater than the sum of the individual stressors).
For example, increases in both temperature and CO₂ concentration have been shown to disproportionately lower the growth rate of some tropical corals.

References
Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.
