Tools of the Trade
Scientists use a variety of tools, from gliders to ships, to measure seawater carbonate chemistry across different spatial and temporal scales.
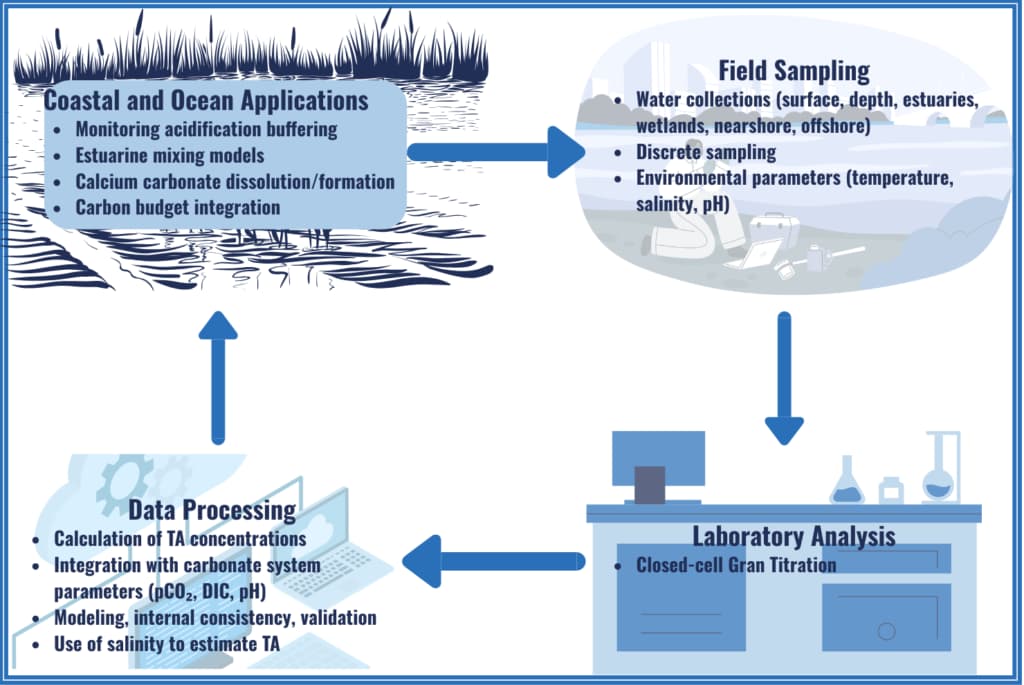
Total Alkalinity (TA)
Total Alkalinity (TA) measures seawater's ability to neutralize acids by accepting hydrogen ions (H⁺), primarily through carbonate ions (HCO₃⁻ and CO₃²⁻) that balance positive charges from other dissolved ions.
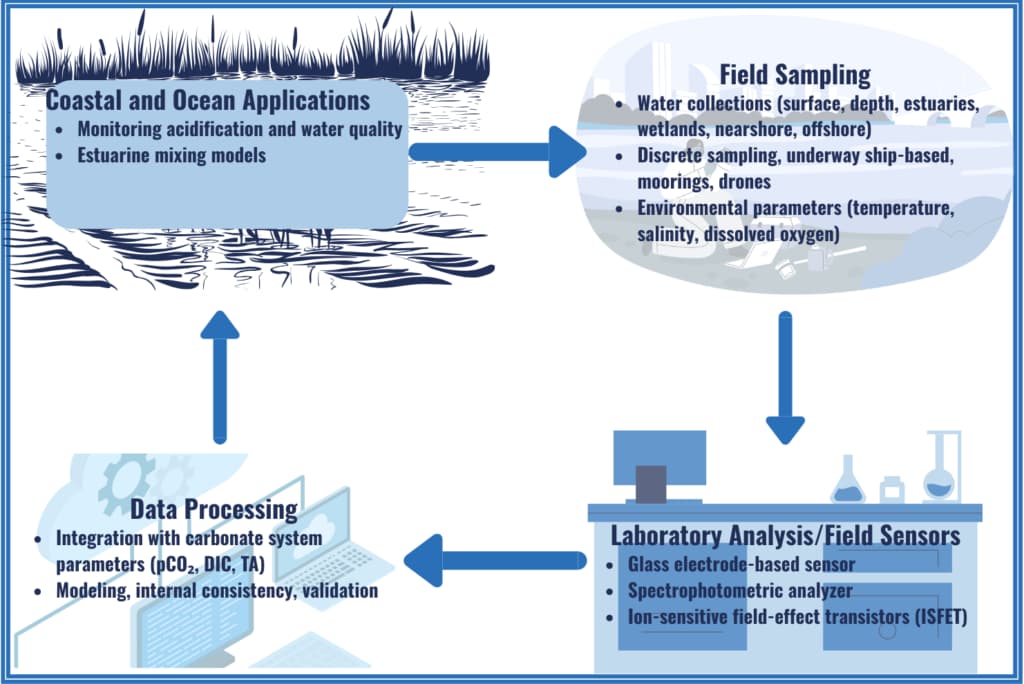
pH
pH measures how acidic or alkaline a solution is based on its hydrogen ion (H⁺) concentration. More H⁺ ions mean higher acidity; fewer H⁺ ions indicate a more basic (alkaline) solution.
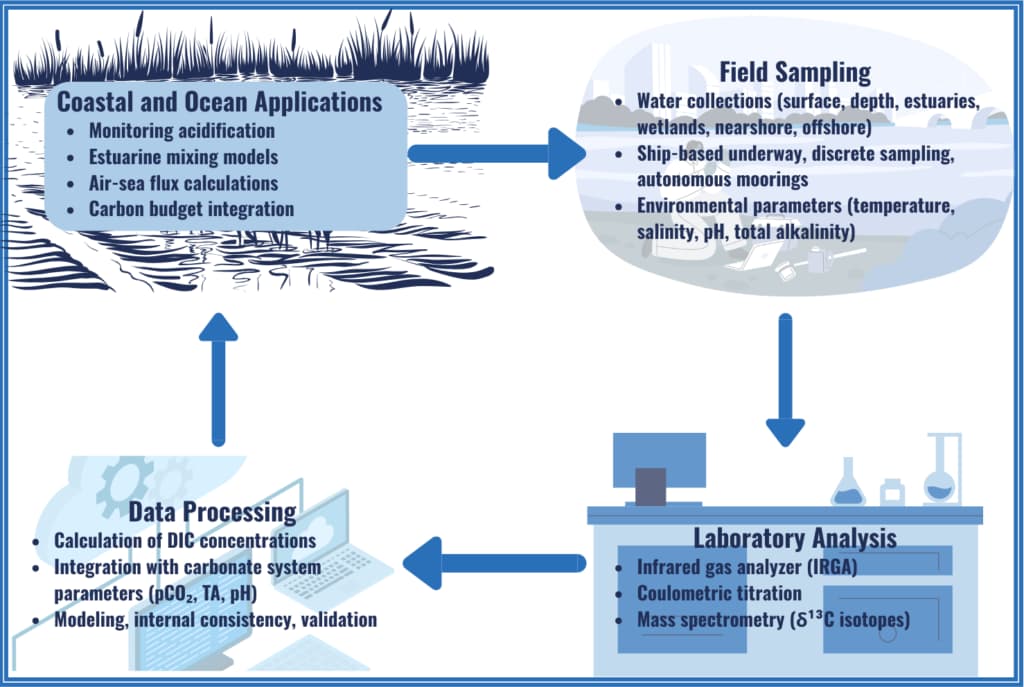
Dissolved Inorganic Carbon (DIC)
Dissolved inorganic carbon (DIC) includes four carbon species formed when CO₂ dissolves in water: aqueous CO₂, carbonic acid (H₂CO₃), bicarbonate (HCO₃⁻), and carbonate (CO₃²⁻).
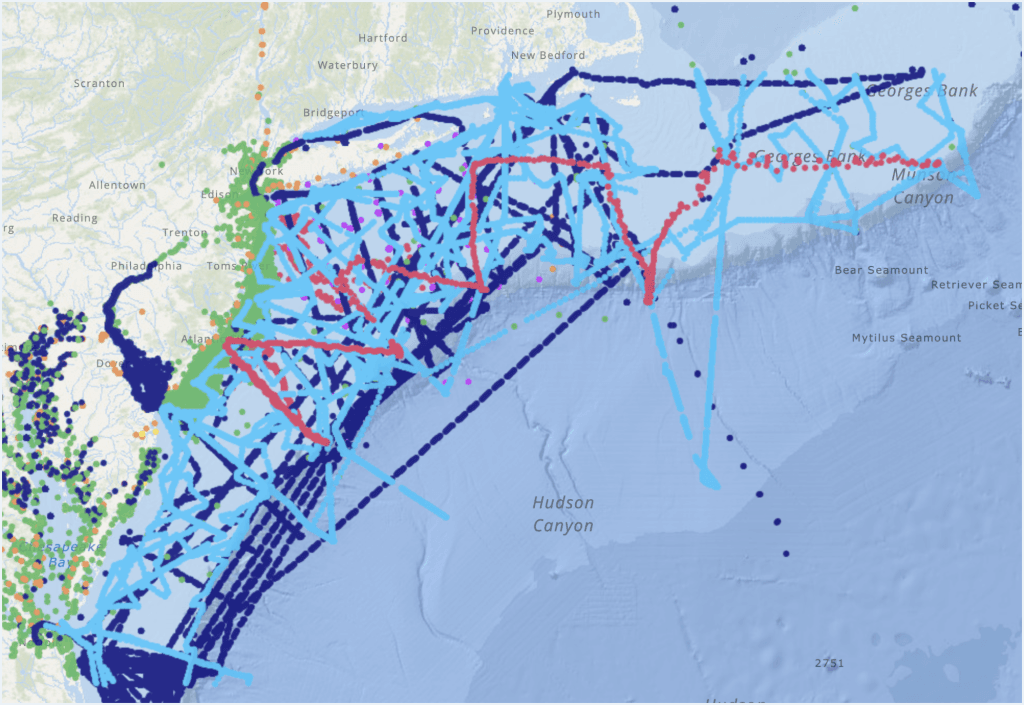
Aragonite Mineral Saturation State (Ω)
Saturation state (Ω) serves as a measure of whether calcium carbonate mineral forms are more likely to dissolve or precipitate under current seawater conditions.
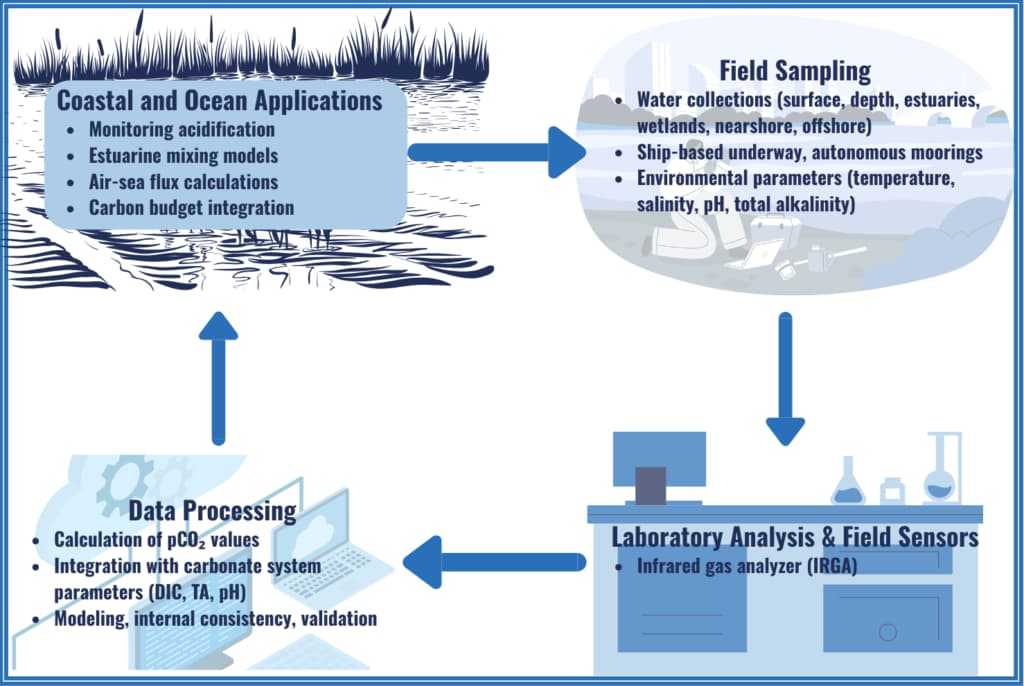
Partial Pressure of Carbon Dioxide (pCO₂)
Get Involved
If you are interested in learning more about MACAN and the work we do, please sign up for our monthly newsletter. You can also read our 2024 to 2028 Work Plan.

The Mid-Atlantic Coastal Acidification Network. All Rights Reserved.
